Fetal Cell Isolation
– Trophoblast Isolation
BioFluidica has deep expertise in fetal cell isolation, isolation of trophoblasts, from maternal blood. We developed *LiquidScan to capture rare cells in bodily fluids, which include circulating tumor cells (CTCs) and circulating trophoblasts in pregnant women’s blood. *LiquidScan provides a cell-based liquid biopsy approach for non-invasive prenatal screening (cbNIPT) to identify genetic abnormalities and inform prenatal and perinatal care.
PRENATAL DIAGNOSTICS
According to data from NIH, approximately 3%-5% of pregnancies in the US are complicated by birth defects or genetic disorders ranging from chromosomal abnormalities to congenital malformations.
Early development for pregnant screening was developed in the 1950’s with ultrasound followed by amniocentesis. Maternal blood contains a host of fetal biomarkers that can be used for screening or diagnosis when combined with improved and reliable cell capture, thus removing risks associated with amniocentesis, relieving patient stress and discomfort.
Over the past two years several studies have demonstrated the feasibility of isolating fetal trophoblasts from a maternal blood sample and highlighted the potential of trophoblast isolation and fetal genome analysis as the first truly diagnostic screen. Isolation methods previously reported generally required large volumes of maternal blood and resulted in very low numbers of fetal cell recovery.
LiquidScan uses maternal blood from a standard blood draw to pass through antibody-surfaced microfluidic chips. Fetal cells present in the maternal blood are affinity captured by the antibodies allowing “background” cells to be washed away. Once washing is complete the fetal cells are released from the bound chips and eluted into collection tubes or slides, within the automated LiquidScan system. It is the unique LiquidScan catch-and-release that makes for the first time cbNIPT possible.
Figure 1. Cytotrophoblast cell capture using BioFluidica’s Liquid Scan. Cells were grown to 80% confluence, detached with trypsin and stained with Cell Trace Green. Stained cytotrophoblasts were spiked into whole blood drawn from healthy donors at 1500, 50, 100, & 8 cells/ml. Trophoblasts were captured from 1ml of spiked blood. The mean capture is shown, error bars represent standard deviation.
The Liquid Scan automated workflow uses preserved maternal blood that is passed through microfluidic chips coated with capture antibodies targeting trophoblasts. The captured cells are then released and eluted from the chip for immunocytochemistry (ICC) staining or FISH for trophoblastic markers.
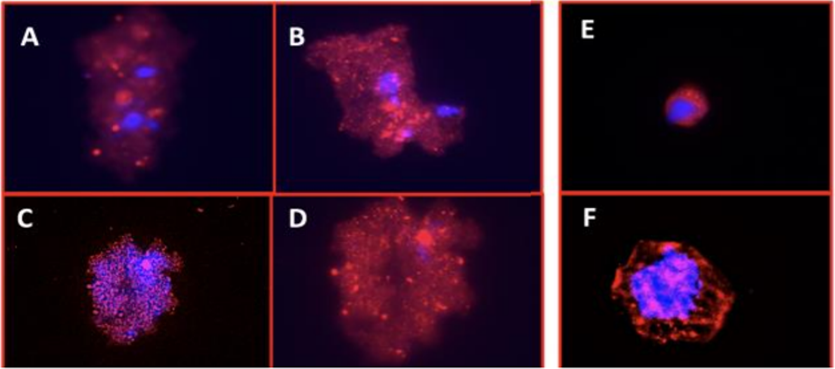
Figure 2. Isolation of trophoblasts from maternal blood. Maternal blood was processed using an optimized microfluidic chip using BioFluidica’s Liquid Scan process. Cells were released and stained with anti-EpCAM antibodies (red) and Hoechst to identify nuclei (Blue). (A-D) Likely multinucleated syncytiotrophoblasts/syncytial knots, (E-F) Likely cytotrophoblasts. The results confirm the presence of fetal cells and using BioFluidica’s platform to rapidly isolate trophoblast cells from a maternal blood samples using a microfluidic chip surfaced with specific capture agents. These studies confirm the isolation of mononucleated (cytotrophoblasts) and multinucleated (syncytiotrophoblasts/syncytial knots) cells. Collectively, these data demonstrate the proof-of-concept of trophoblast cell isolation using a targeting capture agents with BioFluidica’s microfluidic Liquid Scan. The high through-put, automated rare cell isolation method allows large sample processing. The isolated cells are captured in a live, non-fixed state with the potential to be analyzed using whole genome and transcriptome methods, expanding current non-invasive analysis capability.
Schedule a consultation with our research team to discuss your specific needs.
*LiquidScan is for Research Use Only
ABOUT BIOFLUIDICA
BioFluidica is a privately held biotechnology and liquid biopsy company. We have developed a revolutionary liquid biopsy platform allowing for the isolation and analysis of all liquid biopsy biomarkers, including rare cells, extracellular vesicles (EVs; exosomes), and cfDNA, on one fully automated platform. The Biofluidica platform has been clinically tested on nine different cancer and prenatal patient samples and is currently used for a large variety of research applications to advance the field of liquid biopsy. The ultralow sensitivity expands the capability of current liquid biopsy applications. We are expert consultants in exosome isolation, fetal cell (trophoblast) isolation, CTC isolation, and next-generation liquid biopsy. We develop and launch innovative scientific instruments, reagents, and software systems designed to provide exceptional value for scientists, clinicians, and patients.
